Monroe Environmental designs and manufactures high-efficiency Air Strippers for industrial and municipal applications. An Air Stripper removes volatile gaseous compounds dissolved in a contaminated water stream and transfers them to an air stream. Clean water from the Monroe stripper may then be sent to a sump or drain as required. Monroe manufactures Packed Tower Air Strippers as well as a complete line of scrubbers and gas adsorbers that may be used to treat the resulting contaminated air before it is emitted to the atmosphere.
Air Stripping in Wastewater Treatment

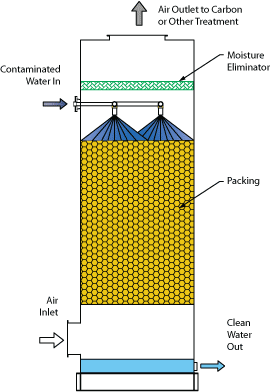
The Monroe Environmental Packed Tower Air Stripper typically uses a counter-flow design with a cylindrical tower containing a specially designed packing media that provides a large surface area for air/water contact. Contaminated water flows down through the packing while air flows upward. VOCs or other contaminants are transferred from the contaminated water stream to the air stream, which then flows out through the top of the tower. Clean water flows out at the bottom of the tower.
- Larger flow rates of contaminated liquid and lower ratio of air flow to liquid flow compared to sieve tray design
- Lower pressure drop than sieve tray air stripper
- Capacities up to 5,000 GPM of contaminated liquid for a single tower
- Efficiency up to 99.9%
- Single and multiple tower designs
- Single-stage or multiple-stage liquid distributors for contaminated water
- Wide range of packing media including rings, saddles, and structured packing
Ideally Suited for Air Stripping of:
- Removal of Volatile Organic Compounds (VOCs) and other compounds with high vapor pressure and low aqueous solubility including:
- Benzene, toluene, and xylenes (BTX)
- Ethylbenzene
- Tetrachloroethylene (PCE)
- Trichloroethylene (TCE)
- 1,2-Dichloroethane (DCE)
- Methyl tert-butyl ether (MTBE)
- Ethylenes
- Ammonia
- Removal of groundwater contaminants from petroleum fuel leakage
- Water treatment plant removal of VOCs from potable water
Air Stripping Theory
The theory behind Air Stripper operation is based on Henry’s Law. Henry’s law states that, at a constant temperature, for a system containing a liquid phase and a gas phase:
p = (kH)(C)
p is the partial pressure of the VOC or other gaseous substance above the liquid. C is the partial pressure (or concentration) of the VOC dissolved in the liquid. kH is the Henry’s Law Constant for the particular gas (solute), liquid (solvent), and temperature. Initially, there is no pollutant gas in the air and a high concentration in the contaminated liquid. So, in order to achieve the equilibrium concentration of gas above the liquid phase predicted by Henry’s law, a portion of the VOC or other dissolved gaseous components will leave the liquid phase and go into the gas phase air stream that exits from the top of the stripper. Air stripping is most effective when the Henry’s law constant (expressed as concentration in gas phase divided by concentration in liquid phase) is large.
Air Stripper Design Features
- Construction available in mild steel, stainless steel, aluminum, FRP, and other materials.
- Wide range of air stripper packing media including rings, saddles, and structured packing
- Complete instrumentation and electrical controls available
- Corrosion resistant AMCA rated fan on the inlet or outlet side of the air stripper
- Corrosion resistant pumps for transport of liquid
- Non-plugging spray nozzles for efficient water distribution and wetting of packing
- Moisture eliminators with chevron, mist pad, or loose fill type designs

Recent Projects
Air Stripper Applications
Resources & Literature
Recent Case Studies
Water | Air Stripper for WTP
Monroe Air Stripper removes 99.97% of the influent contaminants, predominantly TCE from groundwater source for a water treatment plant.